The Future of Colon Cancer Screening is Here.

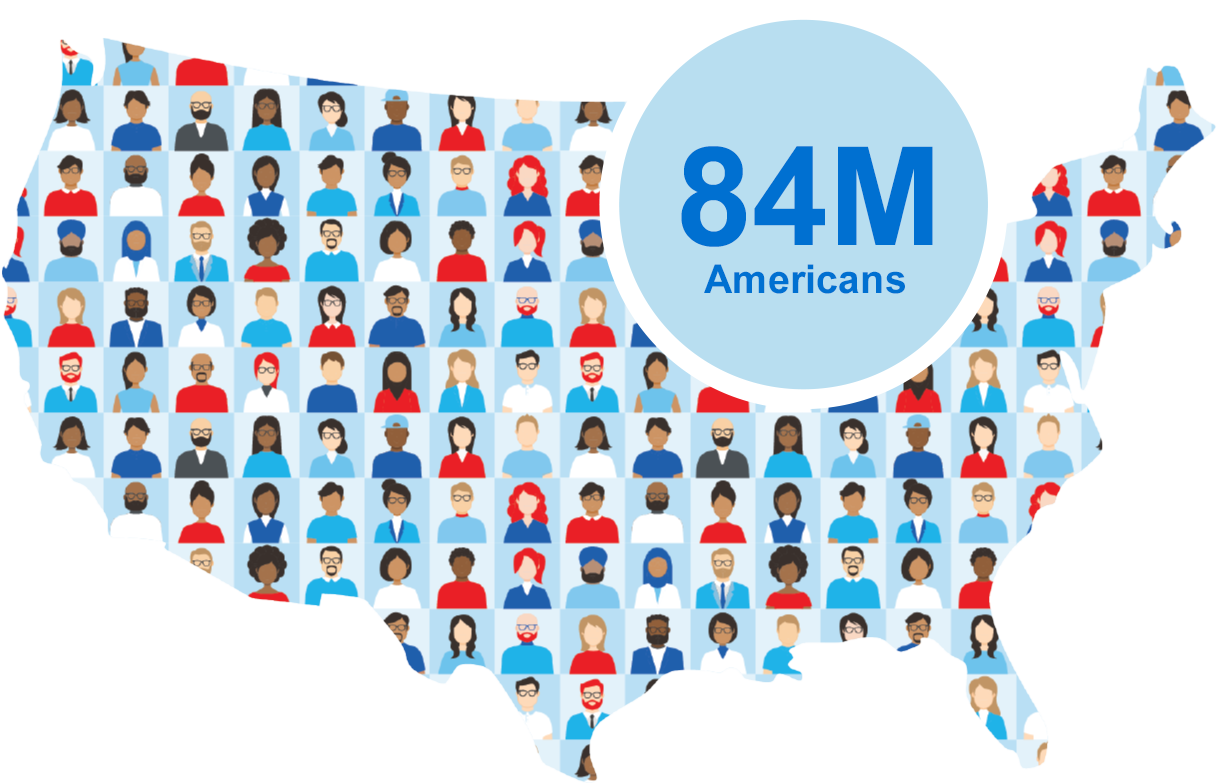
What if $3 million could help protect 84 million Americans?
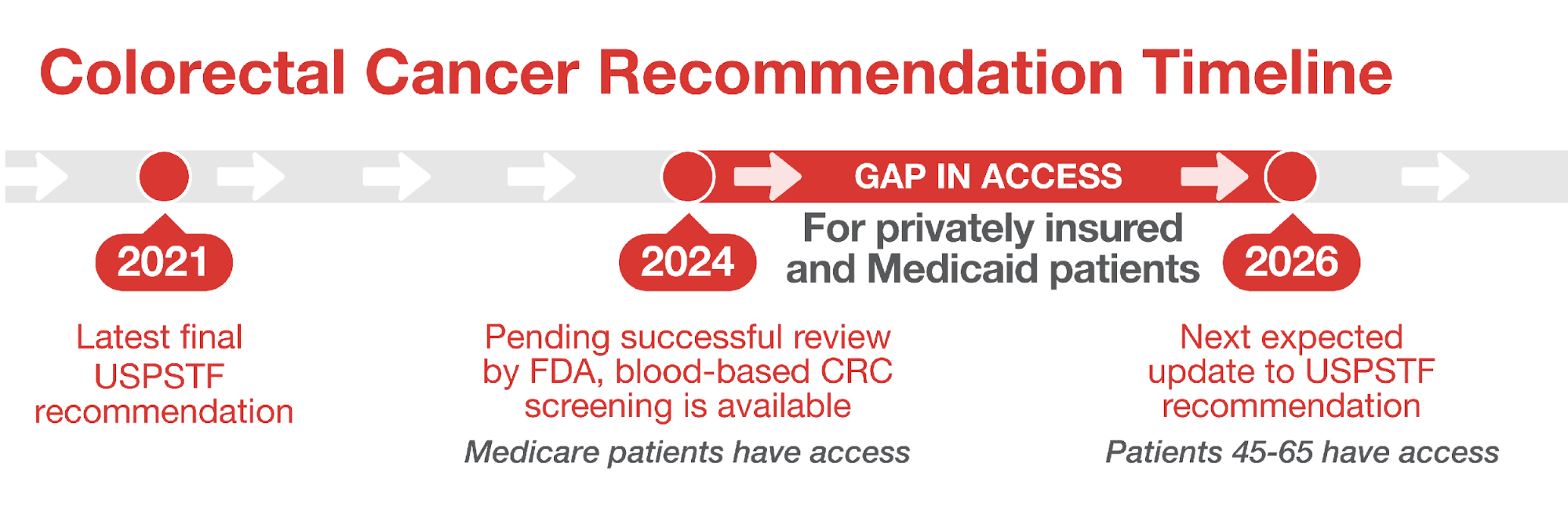
84 million Americans are ages 45-641 – eligible for colon cancer screening but not yet qualified for Medicare¶. CRC screening rates remain stagnant and low with current screening modalities2. Without a ~$3 million increase in funding for the US Preventive Services Task Force (USPSTF)* these individuals could face an out-of-pocket expense for a new blood-based screening test once approved by the FDA – anticipated as early as 2024||.
Cancer screening tests recommended by USPSTF are provided at no cost to the individual. With more funding the USPSTF can update its recommendations so that patients have access to new screening tools without delay.
The USPSTF Lacks the Resources to Keep Pace with Science
The USPSTF aims to review and update existing recommendations every five years, but science sometimes moves faster.
The Task Force is under-resourced and underfunded. With rapid developments in biomedical innovation, the current pace of USPSTF updates could limit adoption of new screening technologies.
The USPSTF Needs More Funding Now
Additional funding can help ensure there is no delay in blood-based CRC screening tests reaching the patients who need them.
The Agency for Health Care Quality and Research (AHRQ)† has repeatedly stated that with additional funding, they could support the Task Force in modifying its processes to keep up with new technologies and changing evidence. More resources could mean more reviews each year.
The President’s latest Budget Proposal‡ included increased funding for USPSTF, but Congress still needs to act.

Additional Resources

Resources to Learn More
- Press Release: Eshoo & Pallone Urge HHS to Reevaluate USPSTF Processes to Better Serve Health Care Needs
- Third Way Briefing: How to Improve Preventative Care
- Third Way Report: Preventive Care: How Do We Know What Works?
- Access to Critical Cancer Screening Tools is At Risk
- Cancer Detection Innovations Stalled by Regulation in Last Mile to Patients

Stakeholder Letters
- Stakeholder Sign-on Letter to Senate Appropriations Committee for FY2024 USPSTF Funding
- Stakeholder Sign-on Letter to House Appropriations Committee for FY2024 USPSTF Funding
- Stakeholder Thank You Letter to President Biden for FY2024 Budget
- Stakeholder Sign-on Letter to OMB for FY2024 President’s Budget

